Optimising malaria vaccine uptake
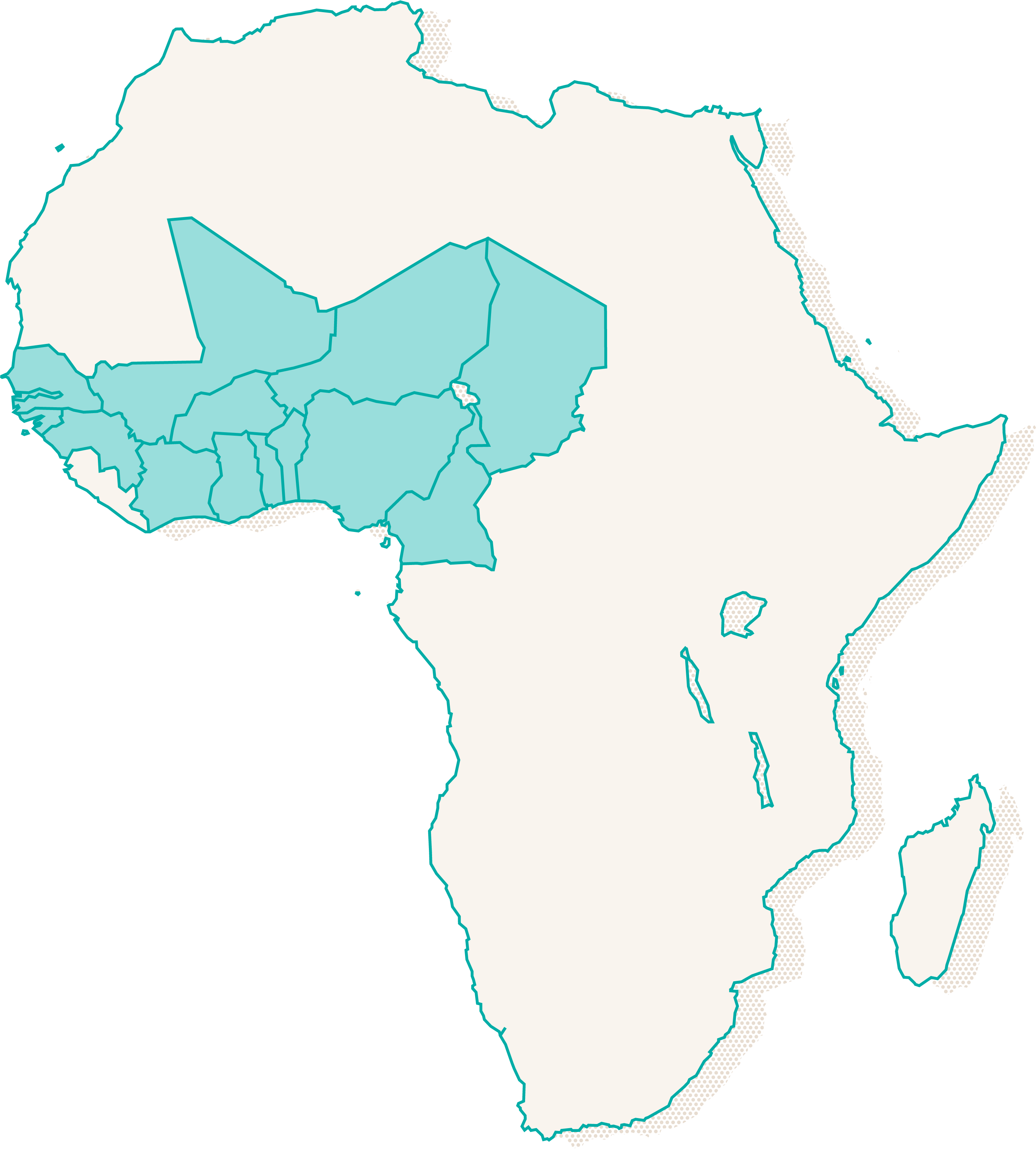
The OPT-MVAC consortium is a partnership between leading African and European research institutions and 14 West and Central Africa countries pursuing malaria vaccine implementation.
Together, we’re working to optimise the delivery and uptake of malaria vaccines in these countries.

Expanding the malaria prevention toolbox
The availability of malaria vaccines is a historic landmark in malaria prevention that could help save tens of thousands of young lives every year. The World Health Organization currently recommends two malaria vaccines (RTS,S and R21 Matrix/M) to help protect children from the disease in malaria-endemic countries. However, a variety of implementation challenges must be addressed to fully realise this potential.
OPT-MVAC consortium partners are supporting the rollout of malaria vaccines in areas with moderate to high transmission rates. This is being achieved through a vaccine implementation research programme, by adapting delivery approaches to local contexts, and sharing data and best practices across our network.
Children under 5
accounted for 76%
of all malaria deaths in 2023
Africa bears the majority of the malaria burden, recording
94% of global cases
in 2023
Powerful trio:
vaccines, chemoprevention and bed nets
are complimentary and can be used together
14 Countries in West and
Central Africa

Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Lorem ipsum dolor sit amet, solet reprimique delicatissimi ex quo, ut cum soluta expetenda. Te sea posse diceret dignissim, rebum oblique mentitum no.
Natum adhuc eirmod et mei, perpetua intellegat cu sed. Ea eum rebum idque. Dicat essent constituam pro ne, quo cu quodsi verterem dissentiet.
Implementation date:
30 August 2025Number of doses administered:
300,679 doses
Project management
Led by Michel Vaillant
WP1 will ensure the project’s objectives, milestones and deliverables are achieved and the workplan is implemented in compliance with the Grant Agreement and the Consortium Agreement. Responsibilities include project support for financial management, logistics, internal communications and coordination, in compliance with European Commission rules and procedures.
Scientific leadership, implementation research, training and research development
Led by Jean-Louis Abdourahim Ndiaye
In collaboration with the Université of Thies, LSHTM and TDR – the Special Programme for Research and Training in Tropical Diseases, WP2 will provide technical support and training to help countries implement research protocols designed to monitor malaria vaccine introduction and identify barriers to uptake and develop mitigation strategies to address potential risks. WP2 will define project priorities and ensure compliance with good clinical practices and international standards. It will provide technical support and training to participating countries to adapt and implement the generic research protocol to their settings and develop mitigation strategies to address any risks.
Epidemiological methods, statistics and
data management
Led by Paul Milligan
WP3 will support reliable and timely data generation to drive improvements in immunisation performance. Activities will include harmonising monitoring and evaluation methods across countries to ensure data are comparable and supporting the ethical conduct of data collection, data integrity and data quality. Support to each country will be provided in-person and via email and WhatsApp, led by partners from LSHTM, with support from LIH, TDR, UIDT and experts in each country.
Safety and pharmacovigilance for
malaria vaccines
Led by Rachida Soulaymani
In collaboration with all consortium partners, WP4 will ensure the selection of administered vaccines in participating countries is based on a positive balance between benefits and risks; that pharmacovigilance systems have the capacity to collect and analyse data to proactively address risks; and that necessary resources are allocated to support the smooth pharmacovigilance operations.
Communications, dissemination and exploitation
Led by André-Marie Tchouatieu
WP5 will share information about the project, its progress and its findings with key stakeholders and engage in activities to facilitate improved access, coverage and trust in vaccines against all preventable infectious diseases
The OPT-MVAC consortium’s workplan consists of five distinct work packages (WPs), each tasked with objectives and deliverables pertaining to the project and its execution.
Subscribe to our newsletter

Disclaimer: Funded by the European Union under Global Health EDCTP3. Views and opinions expressed are, however, those of the author(s) only and do not necessarily reflect those of Global Health EDCTP3 nor its members. Neither of the parties can be held responsible for them.’







